Bioinformatics
The Bioinformatics Core Facility provides support for short- or long-term projects requiring expertise the analysis of biological datasets
Technological innovation and scientific advances are intricately linked and that is why La Jolla Institute puts a big emphasis to provide a first-rate scientific infrastructure and easy access to the latest technology. LJI’s scientific core facilities provide faculty, researchers and students with access to an array of state-of-the-art equipment, technologies, training and expertise to support innovative research. The Institute’s small size allows LJI researchers to interact closely with highly trained experts working in the research support services and to quickly adjust technical applications to the requirements of individual labs.
For more information on accessing our core facilities, please contact our Core Facilities Administrator below.
The Bioinformatics Core Facility provides support for short- or long-term projects requiring expertise the analysis of biological datasets

Clinical research volunteers are crucial partners in the quest for knowledge that will improve the health of future generations.
The LJI Flow Cytometry Core Facility is one of the largest in the San Diego area and provides investigators with state-of-the-art equipment along with the necessary expertise.
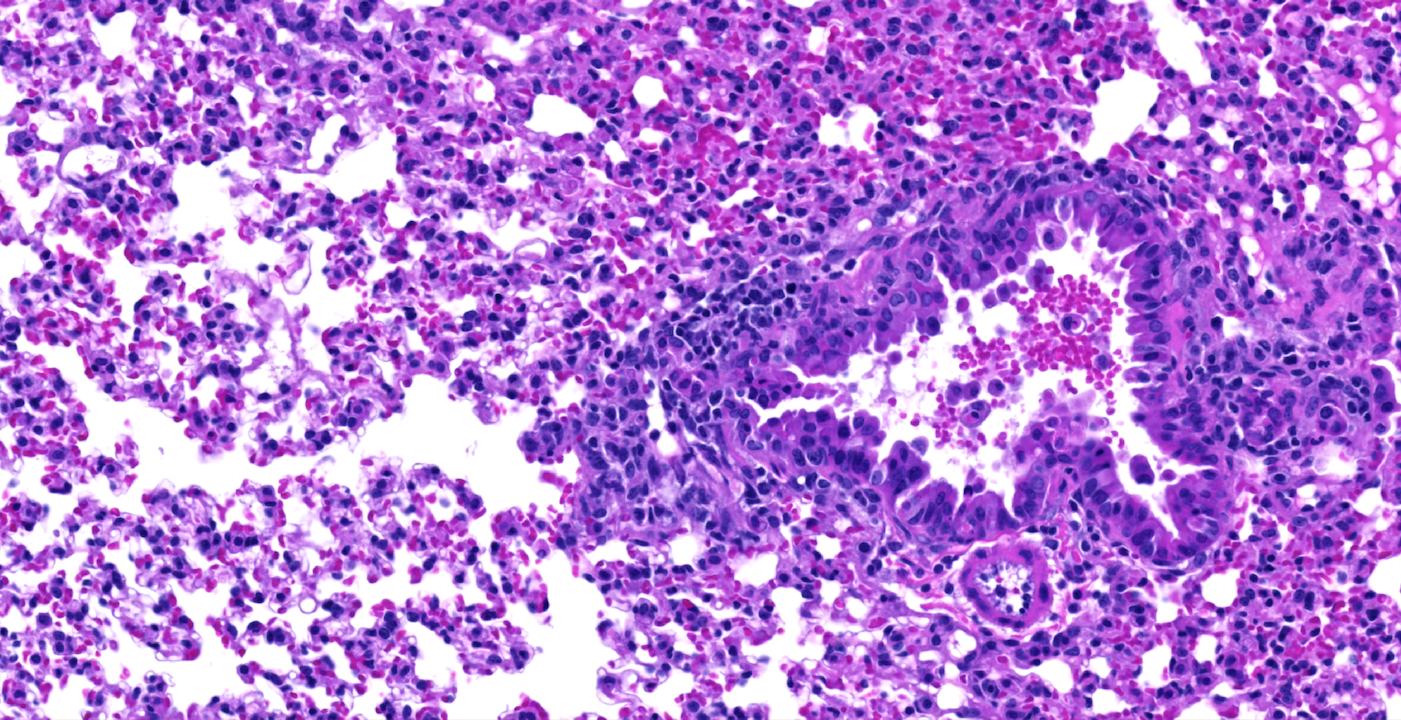
The Histopathology Core offers tailored consulting to intramural and extramural investigators in the analysis of disease pathology.

The immunometabolism Core is a hub within the institute for analysis of metabolism using the instrumentation and methodology that has
The Microscopy and Histology Core Facilities assists local scientists with their imaging needs in a static or dynamic (in vitro/in vivo) scenario and provides the necessary support and training.
The primary goal of the sequencing core facility at LJI is to apply the latest sequencing technology combined with the Institute's expansive bioinformatics capabilities to enrich research at the Institute and beyond.