LA JOLLA, CA—You are mostly but not entirely human. If we crunch the numbers, 8 percent of your genome actually comes from viruses that got stranded there. This viral detritus is a souvenir from our evolutionary past, a reminder that viruses have been with us from the very beginning.
Usually, this 8 percent of your DNA—the viral bits—are kept silent. Scientists call it part of the “dark matter” in your genome.
Now scientists at La Jolla Institute for Immunology (LJI) have published a first look at a key viral protein. In a study published in Science Advances, LJI researchers revealed the first three-dimensional structure of a protein from one of these ancient “human endogenous retroviruses (HERVs).”
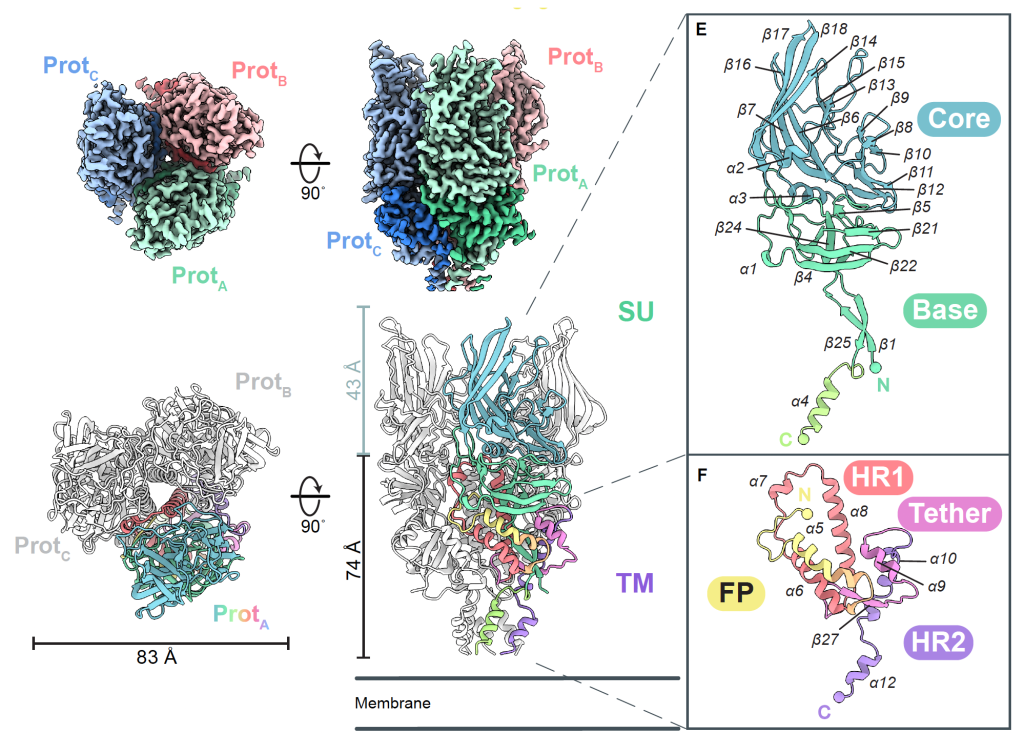
The team mapped the surface envelope glycoprotein (Env), the antibody target of the most active HERV, marking a milestone in structural biology. “This is the first human HERV protein structure ever solved—and only the third retroviral envelope structure solved overall, after human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV),” says Erica Ollmann Saphire, Ph.D., MBA, LJI President, CEO, and Professor.
This discovery opens the door to new strategies for diagnosing and treating disease. Back in the evolutionary past, HERV-K Env proteins studded the outside of the HERV-K retroviruses. But in modern humans, HERV-K Env proteins show up on the surface of certain tumor cells and in patients with autoimmune and neurodegenerative diseases, making them a valuable target for developing novel diagnostics and therapies.
“In many disease states, like autoimmune diseases and cancer, these genes re-awaken and start making pieces of these viruses,” says Saphire. “Understanding the HERV-K Env structure, and the antibodies we now have, opens up diagnostic and treatment opportunities.”
An unexpected “twist”
Until now, HERV proteins had been invisible. They’ve proven too mobile—and too twitchy—to be seen with even the most sophisticated imaging techniques. Solving the structure of HERV-K Env was especially challenging because the LJI team needed to capture the protein’s delicate “pre-fusion” state.
Envelope proteins are full of potential energy—they’re essentially spring-loaded to merge with a host cell to start the infection process. This means pre-fusion proteins are prone to spontaneous switching to their later, post-fusion state. “You can look at them funny, and they’ll unfold,” says LJI Postdoctoral Fellow Jeremy Shek, who spearheaded the study as co-first author with LJI Postdoctoral Fellow Chen Sun, Ph.D.
To study the three-dimensional structure of HERV-K Env, the researchers introduced small substitutions to lock the protein’s structure in place, while preserving its natural shape. Saphire and her team have used this approach before to reveal the structures of key proteins on Ebola virus, Lassa virus, and more. The researchers also discovered and characterized specific antibodies that helped anchor different versions of the viral proteins.
After stabilizing their HERV-K Env structures, the LJI team used a high-resolution imaging technique called cryo-electron microscopy to capture 3D images of HERV-K Env at three key moments: cell surface, in the act of driving infection, and when it locks together with antibodies.
Many viral envelope glycoproteins have a trimer structure, but HERV-K Env is different from anything scientists had seen before, including trimers from other retroviruses. Unlike the shorter, squatter trimers made by HIV and SIV, the HERV-K Env is tall and lean. Further, the protein’s fold—the weaving together of strands and coils that build the working machine—is unlike any other retrovirus.
A new path for clinical research
The new LJI study opens the door to using HERV-K Env to our advantage. Understanding the HERV-K Env structure, and how antibodies target it, may prove useful for developing diagnostic tools or new therapeutics.
For example, many types of cancer cells—from breast cancers to ovarian cancers— but not healthy cells, are dotted with HERV-K Env proteins. This means antibodies against HERV could distinguish cancer cells from healthy cells. As Sun explains, scientists could develop cancer immunotherapies that zero in on HERV-K Env to track down tumor cells. “We can use it as a strategy to specifically target cancer cells,” says Sun.
People with autoimmune diseases such as lupus or rheumatoid arthritis also express HERV-K Env on their cells. Some scientists suspect that patients’ immune cells see these strange proteins and think the body is under attack. Just like during a normal viral infection, their B cells start making antibodies against HERV-K Env proteins.
“Understanding how antibodies recognize these proteins was challenging because there was no structure and precious few good antibodies yet available,” says Saphire.
So the LJI team made their own panel of antibodies to reveal how the immune system can target the different subunits of the molecule in all its different shapes. Once scientists understand how these antibody attacks work, they can try to intervene and stop harmful inflammation.
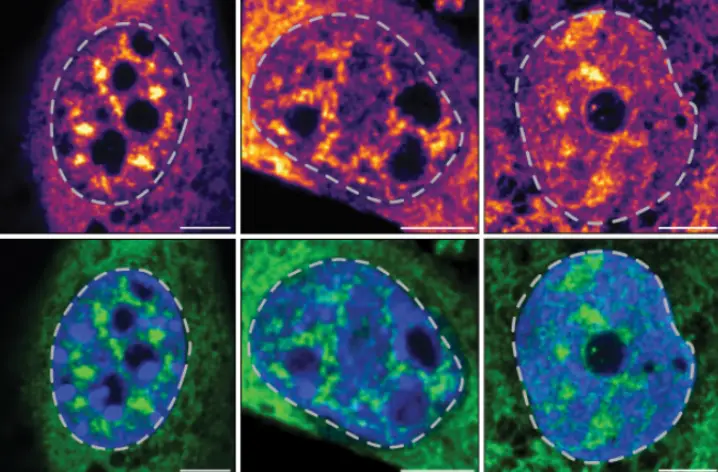
The scientists also tested the idea that their antibodies may also be useful tools for diagnosing many autoimmune diseases. They used the antibodies to try and hunt down immune cells in samples from patients with rheumatoid arthritis and lupus. When Saphire and her colleagues tagged these antibodies with a molecular flag, they were able to quickly detect HERV-K Env on neutrophils, a type of immune cell that can cause inflammation.
“These antibodies marked aberrant HERV display on neutrophils from rheumatoid arthritis and lupus patients, but not healthy controls,” says Saphire.
The interest in HERVs is quickly growing, and scientists are finding more and more diseases where HERV-K Env crops up. “We can really pick whatever disease we’re interested in and go down that route,” says Shek.
These projects may someday advance clinical care—and our fundamental understanding of human biology. After all, we’re all part virus. It’s time to get to know that part of ourselves.
Additional authors of the study, “Human endogenous retrovirus K (HERV-K) envelope structures in pre- and postfusion by cryo-EM,” were Elise M. Wilson, Fatemeh Moadab, Kathryn M. Hastie, Roshan R. Rajamanickam, Patrick J. Penalosa, Stephanie S. Harkins, Diptiben Parekh, Chitra Hariharan, Dawid S. Zyla, Cassandra Yu, Kelly C.L. Shaffer, Victoria I. Lewis, Ruben Diaz Avalos, and Tomas Mustelin,
This study was supported by a Curebound Discovery Grant (13502-01-000-408) and by LJI & Kyowa Kirin, Inc. (KKNA-Kyowa Kirin North America; and a Kirin North America Accelerator Grant [18030-01-000-408]).