LA JOLLA—Every year, more than 68,000 people end up with a clinical case of Japanese encephalitis. One in four of these patients will die. The mosquito-borne virus, which is most common in Southeast Asia, also causes severe neurological damage and psychiatric disorders.
There is no cure for Japanese encephalitis, but there are effective vaccines against Japanese encephalitis virus (JEV). The problem is that JEV’s range is spreading, and more and more people at risk of the disease also live in areas where viruses like Zika are prevalent.
In a new study, published June 5, 2020, in the Journal of Experimental Medicine, scientists at La Jolla Institute for Immunology (LJI) shows that antibodies against JEV are “cross-reactive” and can also recognize Zika virus. Unfortunately, these antibodies can actually make Zika cases more severe. The research, conducted in mice, is the first to show that T cells can counteract this dangerous phenomenon.
“This means we probably need to be developing a vaccine against both viruses that can elicit a good balance of antibodies and T cells,” says Associate Professor Sujan Shresta, Ph.D., who co-led the study in collaboration with Jinsheng Wen, Ph.D., of Ningbo University and Wenzhou Medical University, and Yanjun Zhang, Ph.D., of Zhejiang Provincial Center for Disease Control and Prevention.
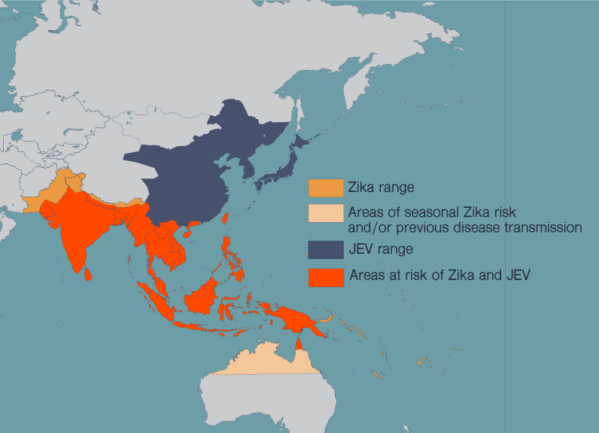
Shresta has spent much of her career studying flaviruses, a family of viruses which includes Zika, JEV, dengue, West Nile virus and yellow fever. These diseases have spread in recent years as more people around the world have moved to cities and climate change has allowed the mosquitoes that carry these diseases to expand their habitat. People in many countries now live at risk of encountering multiple harmful flaviviruses in their lives.
“The immune responses to these viruses are very cross-reactive,” says Shresta. “The problem is that the immune response can be both good and bad.”
In some cases, antibodies against one flavivirus can make a future flavivirus infection even worse by allowing the virus to enter host cells. Shresta and investigators worldwide have shown this process, called antibody-dependent enhancement (ADE), during Zika and dengue infections in animal models that recapitulate severe dengue or Zika disease in individuals with prior exposure to dengue or Zika virus. However, ADE of Zika disease in cases of previous JEV exposure, and the interplay between antibodies and infection-fighting immune cells called CD8+ T cells, had not been studied before.
For the new study, Shresta and her colleagues took antibodies from JEV-infected mice or JEV-vaccinated people and injected them into healthy mice. The healthy mice were then exposed to Zika virus. These mice experienced ADE and had far more severe cases of Zika fever than mice with no antibodies against JEV.
Shresta and her colleagues next focused their attention on CD8+ T cells from JEV-infected mice. They found that CD8+ T cells primed to fight JEV could counteract the harmful effects of cross-reactive antibodies. “These JEV-elicited T cells were indeed able to recognize and get rid of the Zika virus infection,” says Shresta.
In short, the mouse survival rate went up and their viral load went down, thanks to the CD8+ T cells. A future JEV vaccine would need to prompt a similar response from CD8+ T cells to help a person avoid ADE of Zika infection.
Shresta says this work can help shed light on how to fight the whole family of flaviviruses, which includes over 70 different species, and many countries are increasingly dealing with cocirculation of multiple flaviviruses. “Any of these viruses could cause a major, major outbreak,” says Shresta. “We need to look at deploying a combination Zika/JEV vaccine, and we may need to tailor vaccines to particular locations where we know both JEV and Zika pose a threat.”
Shresta adds that research into cross-reactive antibodies and T cell responses is especially important today as scientists investigate whether exposure to common cold coronaviruses can leave a person with any immunity against SARS-CoV-2, the novel coronavirus.
“This provides us with a really good model to learn about immune response,” Shresta says.
The study, titled, “Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis,” was supported by the K.C. Wong Magna Fund of Ningbo University, the Zhejiang Provincial Natural Science Foundation (LY17C010004), the National Natural Science Foundation of China (31870159) and institutional funds from the La Jolla Institute for Immunology.
Additional authors included Zhiliang Duan, Wenhua Zhou, Weiwei Zou, Shengwei Jin, Dezhou Li, Xinyu Chen, Yongchao Zhou, Lan Yang and Yanjun Zhang.
DOI: 10.1084/jem.20192152





