LA JOLLA—A new study from La Jolla Institute for Immunology (LJI) scientists helps answer the question: how long does immunity against COVID-19 last in vaccinated people?
As they report in Science, a low dose of the Moderna vaccine lasts for at least six months, and there is no indicator that vaccinated people will need a booster shot.
“This time point is critical because that is when true immune memory has formed,” says LJI Research Assistant Professor Daniela Weiskopf, Ph.D., who co-led the study with LJI Professors Alessandro Sette, Dr.Biol.Sci., and Shane Crotty, Ph.D.
In fact, while the Moderna COVID-19 vaccine (mRNA-1273) led to strong CD4+ (helper) T cell, CD8+ (killer) T cell and antibody responses for at least six months after clinical trial participants were fully vaccinated, it is likely that the immune response could last much longer. The researchers also show that this strong immune memory lasted in all age groups tested, including in people over age 70, a demographic especially vulnerable to severe COVID-19.
“The immune memory was stable, and that was impressive,” adds Crotty. “That’s a good indicator of the durability of mRNA vaccines.”
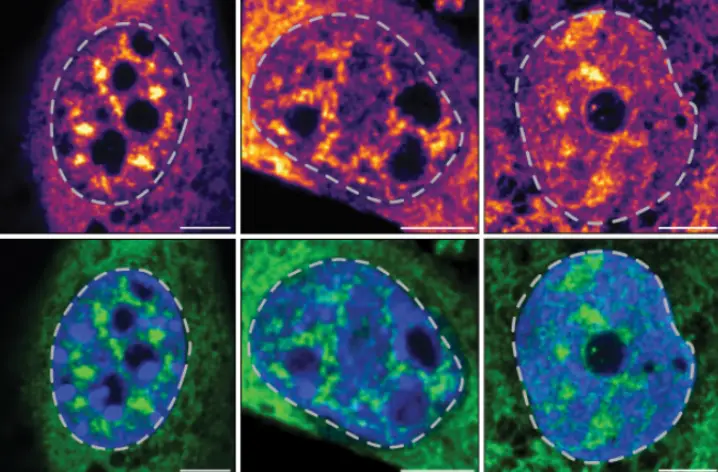
Comparing Moderna vaccine to natural immunity
The researchers compared recovered COVID-19 patients to vaccine trial participants who received a 25-microgram dose of the Moderna vaccine during the phase 1 clinical trials (supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health).
“We wanted to see if a quarter of the dose is able to induce any immune response,” says study first author Jose Mateus Triviño, Ph.D., a postdoctoral fellow at LJI. “We had the opportunity to receive the samples from the original Moderna/ NIH phase 1 trial participants who had received two 25-microgram injections of the vaccine, 28 days apart.”
This vaccine dose is a quarter of the 100-microgram Moderna dose given emergency authorization by the Food and Drug Administration (FDA). While researchers don’t know whether this smaller dose is as effective as the standard dose, this new study shows that the T cell and antibody response in the smaller dose group is still strong.
In fact, the researchers found that the Moderna vaccine spurs an adaptive immune response to the SARS-CoV-2 spike protein (a key target) nearly identical to the immune system’s response to a natural SARS-CoV-2 infection. “The response is comparable,” says Weiskopf. “It’s not higher and it’s not lower.”
The new study does not show that a lower dose of the Moderna vaccine provides the same protection as the standard dose. “It would take a clinical trial to tell you how protective the lower dose is,” says Crotty.
Common cold viruses do prep the immune system
The new research also shows the power of “cross-reactive” T cells. In a 2020 Science study, the LJI team showed that T cells in people who had recovered from common cold coronaviruses could respond to the novel coronavirus, SARS-CoV-2. At the time, they didn’t know whether this cross-reactivity could actually protect against COVID-19.
“Understanding the role of cross-reactive T cells is important because T cells play an important role in the control and resolution of COVID-19 infections,” says Sette.
For the new study, the researchers found that people with cross-reactive T cells had significantly stronger CD4+ T cell and antibody responses to both doses of the vaccine.
“If you have this immune reactivity, your immune system may kick in faster against the virus,” says Sette. “And multiple studies have shown that how quickly the immune system reacts is key.”
Moderna vaccine activates “killer” T cells
The team also filled in an important gap in COVID-19 vaccine research. Until now, many studies had shown an effective CD4+ T cell response to the Moderna vaccine, but CD8+ T cell data was lacking.
“We know naturally infected and recovered people develop excellent CD8+ T cell responses against SARS-CoV-2; however, there was concern about the generation of CD8+ T cells by mRNA vaccines,” says Mateus Triviño.
The new study shows a strong CD8+ T cell response to the low dose Moderna vaccine, similar to the response after a patient fights a natural SARS-CoV-2 infection, says Sette, a renowned T cell expert.
“We see a robust CD8+ T cell response—and we showed that using multiple assays,” adds Weiskopf.
Coming up:
Will this same vaccine durability hold true for the other types of COVID-19 vaccines? Weiskopf and her colleagues are investigating. In the meantime, Weiskopf says real-world data suggest immune memory does last.
“The people in the hospitals are the ones not vaccinated,” she says.
The researchers are also interested in how the durability of the Moderna vaccine compares with other COVID-19 vaccines in use.
This study, “Low dose mRNA-1273 COVID-19 vaccine generates durable T cell memory and antibodies enhanced by pre-existing crossreactive T cell memory,” was supported by the National Institutes of Health’s (NIH) National Institute for Allergy and Infectious Diseases (NIAID; grants AI142742 and AI135078), NIH contract Nr. 75N9301900065 and LJI Institutional Funds.
This work used samples from the phase 1 mRNA-1273 study (NCT04283461), sponsored and primarily funded by NIAID, in part with federal funds from the NIAID under grant awards 5 UM1AI148373, to Kaiser Washington; UM1AI148576, UM1AI148684, and NIH P51 OD011132, to Emory University; NIH AID AI149644, and contract award HHSN272201500002C, to Emmes. Funding for the manufacture of mRNA-1273 phase 1 material was provided by the Coalition for Epidemic Preparedness Innovation.
Additional study authors include first author Jose Mateus, Jennifer M. Dan, Zeli Zhang, Carolyn Rydyznski Moderbacher, Marshall Lammers and Benjamin Goodwin.
###