LA JOLLA, CA—Scientists can finally hunt down a harmful kind of human T cell, thanks to new research led by scientists at La Jolla Institute for Immunology (LJI) and the Medical College of Georgia (MCG) at Augusta University.
Immune cells called ex-T regulatory cells (exTregs) tend to be rare in the body and, so far, impossible to detect in human samples. The new study gives scientists a reliable way to find human exTregs and provides a window into how exTregs contribute to inflammation and cardiovascular disease.
The researchers used fluorescent tags to track down exTregs in mice prone to atherosclerosis. They then identified specific markers that made these exTregs special. They transposed these findings to humans to detect exTregs in human blood.
“These cells are likely causing damage,” says LJI Postdoctoral Researcher Payel Roy, Ph.D., co-first author of the new Nature Immunology study. “Now we have the potential to use these biomarkers and screen for these cells in human blood.”
“Identifying exTregs in humans opens up more opportunities for research since patient material—like blood samples—are readily available,” adds study senior author Klaus Ley, M.D., co-director of MCG’s Immunology Center of Georgia.
Crazy ex-T regulatory cells
T cells do many jobs in the body. Some T cells have the job of alerting other immune cells to danger or destroying infected host cells. These T cells are the fighters, the offensive lineup. T regulatory cells (Tregs) have the important job of stopping the other T cells from releasing too many inflammatory, or cytotoxic, molecules as they fight infection. The Tregs are like the referees.
In previous studies, Ley and his LJI colleagues found that some T cells contribute to atherosclerosis by attacking a molecule called apolipoprotein B (APOB), the main component in the “bad” cholesterol that builds up into dangerous plaques in the arteries. These T cells ramp up their attacks as atherosclerosis worsens, likely adding to inflammation in the arteries. The weird thing is that these T cells look a lot like the normally helpful Tregs.
The new study reveals the true identities of these cells: they are exTregs. ExTregs are like zombie Tregs. They’ve gone through a genetic “deprogramming” and lost their ability to help regulate inflammation.
Scientists don’t know exactly why exTregs develop, but the phenomenon may happen when the body misses the mark in an attempt to adapt to chronic disease.
“When you have too much chronic inflammation, say, for example, in heart disease, your body is highly stimulated and may be rewired to address the situation,” says LJI Postdoctoral Researcher Antoine Freuchet, Ph.D., who served as co-first author of the new study. “Because of that, this kind of Tregs, whose purpose was to keep the bad guys in check, turn bad themselves. Instead of controlling the inflammatory guys, they acquire inflammatory properties.”
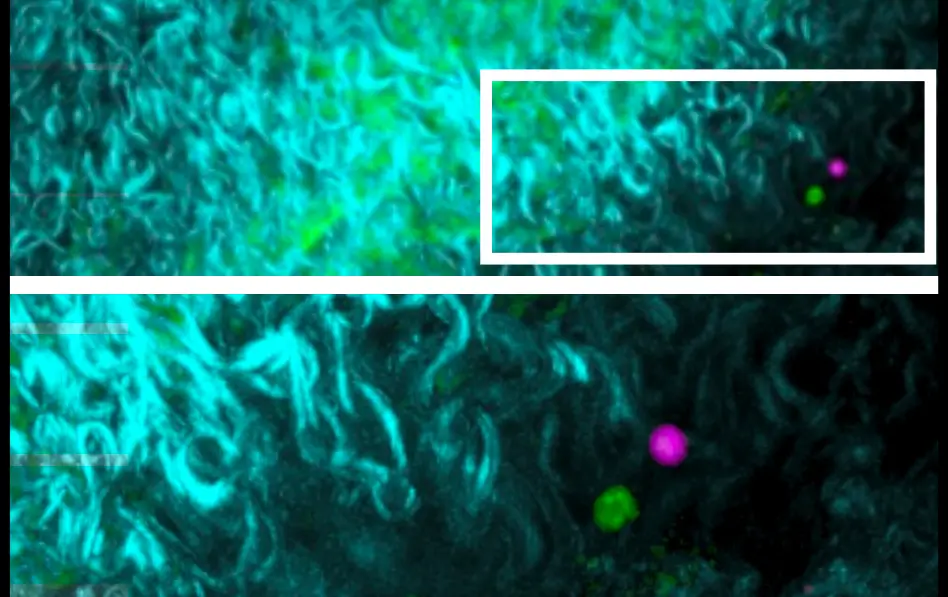
Red cell, green cell
The new study had to begin in mice. “ExTregs have been identified in mice for a long time, but there were no tools to find them in people,” says Ley. “In mice, you can use genetic manipulation to label cells, but you cannot do that in people.”
For the study, the researchers tagged both Tregs and harmful exTregs in a mouse model prone to atherosclerosis. These fluorescent red and green tags glowed when Tregs were functioning normally. A switch from green and red to only red revealed exTreg development in real time.
The team then took organ samples from the mice and used a technique called flow cytometry to detect the green and red tags to sort the Tregs from the exTregs. The researchers used techniques called bulk RNA sequencing to learn more about these cells.
The sequencing highlighted vast differences in gene expression and showed that exTregs make a distinct set of genes that sets them apart from Tregs. At last, the scientist could detect specific exTreg markers.
Detecting exTregs in humans
Next, the researchers used the exTreg markers found in mice to transpose them to a single cell RNA sequencing performed on human blood samples. Through this process, they successfully identified biomarkers for human exTregs. They studied human blood samples provided by researchers at the University of Virginia and by LJI’s John and Susan Major Center for Clinical Investigation.
The researchers were thrilled to find that the exTreg biomarkers they’d seen in mouse samples were also relevant for human exTregs. They discovered that exTregs from humans with atherosclerosis are potentially more potent.
“Now that we can detect ex-Tregs in the blood, we can fish them out specifically and know who they are—know their molecular fingerprints,” says Roy.
Going forward, the researchers hope to use exTreg biomarkers to detect and study the roles of these cells in other chronic health conditions, such as in patients with autoimmune diseases. Roy and Freuchet are also interested in studying samples from the same individual patients taken over time. How might exTreg biomarkers change as atherosclerosis changes? Would they see decreased signs of exTregs if a patient was put on an effective medication?
“This could be a powerful tool for future studies,” says Freuchet.
Additional authors of the study, “Identification of human exTregs as CD16+CD56+ cytotoxic CD4+ T cells,” include Sujit Silas Armstrong, Mohammad Oliaeimotlagh, Sunil Kumar, Marco Orecchioni, Amal J Ali, Amir Khan, Jeffrey Makings, Qingkang Lyu, Holger Winkels, Erpei Wang, Christopher Durant, Yanal Ghosheh, Rishab Gulati, and Felix Nettersheim.
This research was supported by the National Institutes of Health (grants P01 HL136275 and R35 HL145241) an American’s Heart Association Career Development Award (#941152), the Neven-DuMont Foundation, and a Deutsche Forschungsgemeinschaft Fellowship (NE 2574/1-1).
DOI: 10.1038/s41590-023-01589-9
###




