LA JOLLA, CA, & PITTSBURGH, PA—New research from the University of Pittsburgh School of Medicine and La Jolla Institute for Immunology, published today in Nature Microbiology, reveals an opportunity for developing a therapy against cytomegalovirus (CMV), the leading infectious cause of birth defects in the United States.
Researchers discovered a previously unappreciated mechanism by which CMV, a herpes virus that infects the majority of the world’s adult population, enters cells that line the blood vessels and contributes to vascular disease. In addition to using molecular machinery that is shared by all herpes viruses, CMV employs another molecular “key” that allows the virus to sneak through a side door and evade the body’s natural immune defenses.
The finding might explain why efforts to develop prophylactic treatments against CMV have, so far, been unsuccessful. This research also highlights a new potential avenue for the development of future antiviral drugs and suggests that other viruses of the herpes family, such as Epstein-Barr and varicella-zoster virus (which causes chickenpox and shingles), could use similar molecular structures to spread from one infected cell to the next while avoiding immune detection.
“If we don’t know what weapons the enemy is using, it is hard to protect against it,” said senior author Jeremy Kamil, Ph.D., associate professor of microbiology and molecular genetics at Pitt. “We found a missing puzzle piece that represents one possible reason why immunization efforts against CMV have been unsuccessful.”
In the United States, approximately one in every 200 babies is born with congenital CMV infection. Of the babies infected, one in five will have birth defects, such as hearing loss, or go on to have long-term health challenges. For most adults, CMV infections are asymptomatic. But a CMV infection during pregnancy presents significant health risks to the developing child and could be deadly for people who are immunosuppressed, including organ transplant recipients.
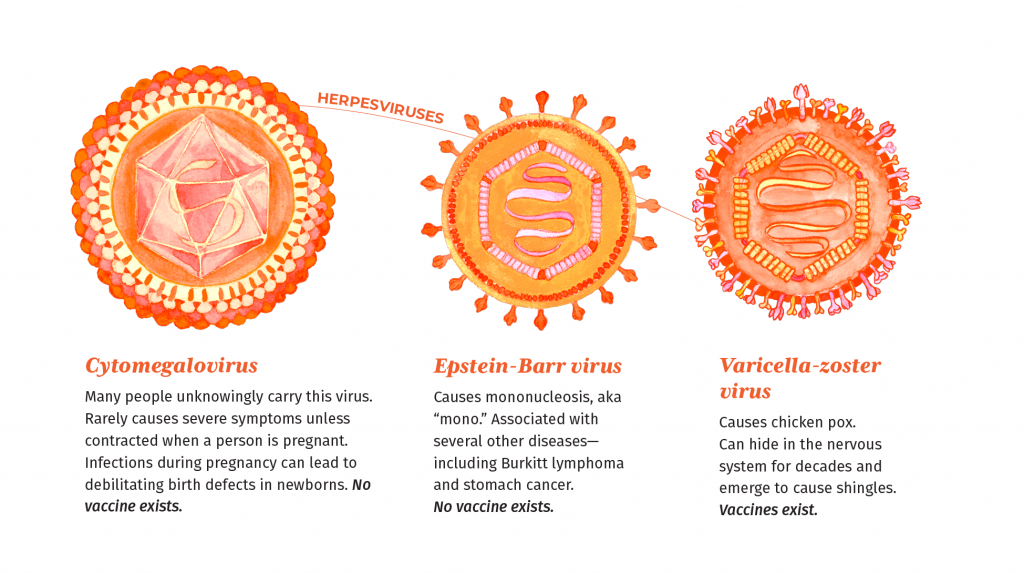
Because of the large size of its genome and its complicated molecular machinery, CMV long evaded attempts to develop prophylactic treatments. Similar to other herpes viruses, CMV relies on a protein called gH to enter cells of the vessel lining. But unlike other herpes viruses, which use a protein partner called gL to facilitate infection, the new study found that CMV replaces gL with another partner called UL116 and recruits a protein called UL141. The resulting complex of gH-UL116-UL141, called GATE by the authors, then becomes an alternative tool for breaking into cells lining the blood vessels and causing internal damage while simultaneously preventing the body’s own immune system from recognizing the signs of infection.
The newly discovered GATE could become a potential vaccine target for CMV and other herpes viruses.
“Previous attempts to generate a CMV vaccine have failed, but that was before we identified the GATE complex. We hope that new strategies targeting GATE will improve our chances to combat CMV infection, and also perhaps cleanse our bodies of this lifelong infection,” said Chris Benedict, Ph.D., Associate Professor at La Jolla Institute for Immunology and co-senior author of the study with Kamil and LJI Professor, President & CEO Erica Ollmann Saphire, Ph.D., MBA. “If we can develop antiviral drugs or vaccines that inhibit CMV entry, this will allow us to combat the many diseases this virus causes in developing babies and immune-compromised people.”
Other authors of the study, “The GATE glycoprotein complex enhances human cytomegalovirus entry in endothelial cells,” are Michael Norris, Ph.D., of the University of Toronto; Lauren Henderson, Mohammed Siddiquey, Ph.D., both of Louisiana State University Health Shreveport; and Jieyun Yin, Ph.D., Kwangsun Yoo, Ph.D., Simon Brunel, Ph.D., and Michael Mor, Ph.D., all of La Jolla Institute for Immunology.
This research was supported by the National Institutes of Health (grants AI11685, AI139749, AI101423 and T32HL155022) and by ARPA-H APECx contract 1AY1AX000055.
DOI: 10.1038/s41564-025-02025-4
# # #
About the University of Pittsburgh School of Medicine
As one of the nation’s leading academic centers for biomedical research, the University of Pittsburgh School of Medicine integrates advanced technology with basic science across a broad range of disciplines in a continuous quest to harness the power of new knowledge and improve the human condition. Driven mainly by the School of Medicine and its affiliates, Pitt has ranked among the top recipients of funding from the National Institutes of Health since 1998. In rankings released by the National Science Foundation, Pitt is in the upper echelon of all American universities in total federal science and engineering research and development support.
Likewise, the School of Medicine is equally committed to advancing the quality and strength of its medical and graduate education programs, for which it is recognized as an innovative leader, and to training highly skilled, compassionate clinicians and creative scientists well-equipped to engage in world-class research. The School of Medicine is the academic partner of UPMC, which has collaborated with the University to raise the standard of medical excellence in Pittsburgh and to position health care as a driving force behind the region’s economy. For more information about the School of Medicine, see www.medschool.pitt.edu.