IF FINDING A WAY TO PREVENT CARDIOVASCULAR DISEASE IS ONE OF THE HOLY GRAILS OF MEDICAL RESEARCH, PROFESSOR LEY MAY BE FURTHER ALONG THAN MOST IN THAT QUEST.

A vascular biologist-turned-immunologist, Dr. Ley has made huge strides by proving his vaccine successfully reduces atherosclerotic plaque in mice and he believes the same process will work in humans. The much-honored Dr. Ley was born and raised in Germany and received his medical degree from the Julius-Maximilians-Universität, Würzburg, Germany, in 1982. He trained as a postdoctoral researcher at the Freie Universität Berlin, to which he returned after a short stint as a visiting research scientist at the University of California, San Diego. In 1994, he joined the faculty of the University of Virginia where he served as Director of the Robert M. Berne Cardiovascular Research Center from 2001 until 2007 when he was recruited to LJI.
How did you go from studying to be a physician to ending up as an internationally known biomedical researcher?
One day during a high school physics class my teacher said, “Klaus, you’re going to be a scientist.” Being a teenager and somewhat rebellious, I said, “No, I’m going to be a doctor” and I went to medical school. He was right, though. In my third year I started doing lab research and realized I’m very curious and love learning about all things in life, especially the scientific world. I knew then I didn’t want to be a doctor and take care of patients but I still went ahead and completed my medical degree, knowing that every position I pursued after that would be pure research.
How were you able to make that transition?
The challenge was that while medical school was an excellent education, and to this day continues to provide me with valuable insight into what the ultimate goal of my research is, the training is somewhat superficial in that you learn a little about everything but nothing in depth. It lacked the rigor I would have gained had I pursued a research Ph.D. I knew had some catching up to do, so the solution I came up with was to do two postdocs: the first, in Berlin, helped me learn the basics, almost like a Ph.D., and the second at UC San Diego, prepared me to become a faculty member at a major research center.
Your Berlin postdoc was before the Iron Curtain fell, wasn’t it?
Yes, and I almost didn’t take it. You had to drive four hours through East Germany to get to West Berlin and they hold onto your passport until you return. It was November and in the northern latitudes the nights are long and wet. It was all very dreary and disconcerting and I didn’t know if I could handle it. I decided to take the plunge and it turned out great. The research on microcirculation was narrow but deep and it gave me a real foundation for becoming a vascular biologist.
You leveraged that foundation into a remarkable career that culminated with you heading up University of Virginia’s Cardiovascular Research Center in the early 2000s. You were a pioneer in vascular biology, published countless papers, and won virtually every major award in your field. Why did you then take another right turn, this time into immunology?
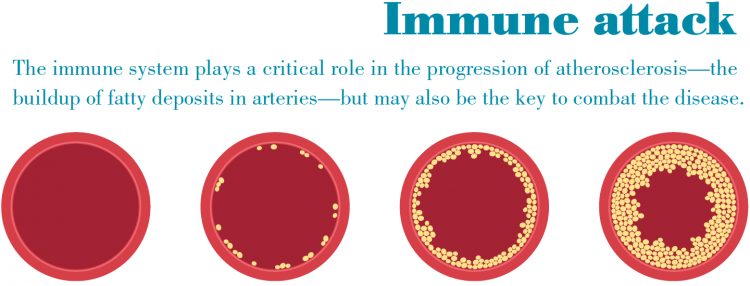
By that time our center was at the leading edge of discovering the inflammatory nature of atherosclerosis. But I also was beginning to believe there was an important connection between the immune system and its role in causing and controlling inflammation. I sensed we might be able to use the immune system to manipulate inflammation with the goal of possibly preventing or curing disease. The problem was that I was an expert in inflammation biology but I knew I needed more knowledge and experience on how the immune system works. I knew there was no better place to gain that knowledge than at one of the world’s leading immunology institutes, so it was an easy decision to come to La Jolla Institute.
In the 13 years since, you and your team have made a number of important discoveries— including your work on neutrophils and olfactory receptors—but the most important is probably your research on an atherosclerosis vaccine. How close is it to becoming a reality?
The good news is we’re making excellent progress, having already proven the vaccine works in mice. Even so, we’re realistic enough to understand that we’re still a number of years away from proving the vaccine works in humans, which is a far more complicated process. We are confident in our basic discovery that identified the type of immune cells (CD4 T cells) that orchestrate attacks on the artery walls, and that these immune cells act as if they have already seen the antigen that leads them to launch the attack. These immune cells seem to remember the molecule brought forth by the antigen-presenting cells, and this immune memory is what allowed us to create a vaccine prototype.

What is the biggest challenge in getting the vaccine to work in humans?
Let’s start with the fact that mice and humans are completely different species and that the human body and its immune system is far more complicated. That makes mice limited in value as a disease model, but it’s nevertheless a critically important starting point. Right now we’re focusing a lot of our attention on a real challenge, something we call “the switch.” That means that while the vaccine might be anti-inflammatory for most everyone we would give it to, there is a chance in some people the process could switch and actually turn pro-inflammatory and do real harm to the patient. Until we
figure out why that switch occurs and how to prevent it, we’ve still got a ways to go before we can call the vaccine safe, effective, and durable.
If you are able to perfect the vaccine, what is its potential value?
Based on how much we were able to reduce atherosclerosis in mice, we believe we might be able to achieve a 50 percent reduction in atherosclerosis in humans. That’s why we’re working so hard on it because we know it would have such a huge impact on human health. We believe it could literally save millions of lives around the world and it would definitely be the highlight of my career. Ironically, it would bring me full circle from my medical training in that I would actually be helping heal patients.

Looking back on that career, do you think you made the right decision to become a researcher?
Absolutely. My life in scientific research has been everything I hoped it would be and much more. I love coming up with hypotheses and then designing experiments to kill those hypotheses as quickly as possible to get down to what is true and verifiable. That’s the best and only way to conduct science. If you fall in love with your hypotheses and design irrelevant experiments that never challenge your theories, you’ll never make any progress. I’ve been a scientist for nearly 40 years and I actually see that as a huge advantage in helping me create groundbreaking research. When you first start out you want to be very specific and focused in a small niche so you get your grants and get taken seriously by your fellow scientists. But now, after having gathered a huge amount of knowledge over the years, I have the wide experience that enables me to see science and research problems on a big picture level. That’s really valuable because real-life diseases require broad approaches to attack, and I’m fully aware there is no single magic bullet that will suddenly solve a complex scientific problem.
How do you relax when you’re not in the lab?
I enjoy cooking and spending time working on my classic 1964 Mercedes 230SL convertible. I also like long distance running. I just finished a half marathon in December and will run another one in April. Going on long runs is a good time to think scientifically. I don’t wear headphones or take my phone so I can concentrate. A lot of big ideas or breakthroughs to research problems have come when I’m out there on the road.


