Your immune system is you.
You were born with the inklings of immune protection, and this protection grew and grew as your body learned to fight off bacteria and viruses.
Your T cells are key to immune protection. T cells race through your bloodstream, and some nestle into your organs. These T cells are supposed to follow a basic rule: Don’t attack this body. This body is you. It is self.
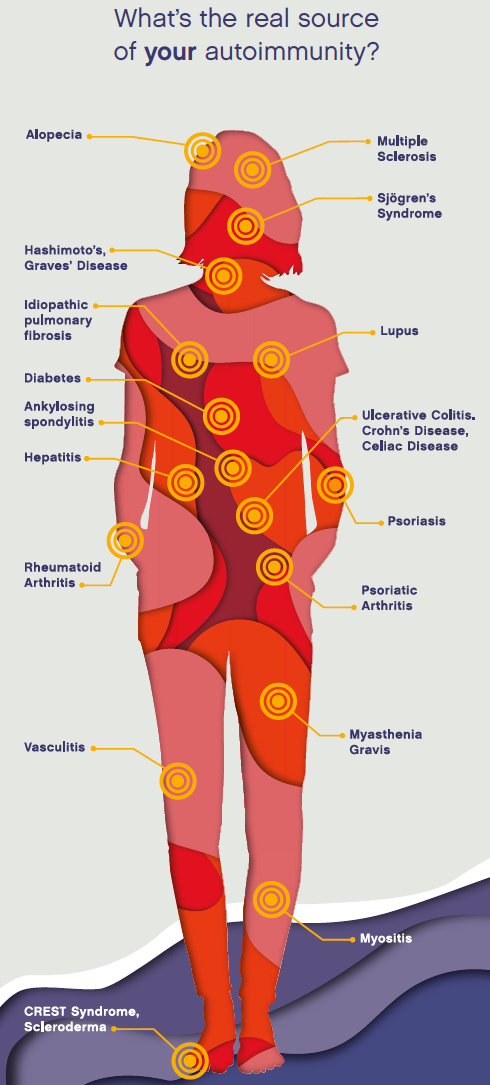
And yet millions of people suffer from autoimmune diseases, where T cells start attacking the body’s own tissue. There are around 80 autoimmune diseases. Each has different targets—the skin, the lungs, the bowel—but they have much in common.
“In all autoimmune diseases, you have a revved-up immune system that is responding too well, or it is responding to something it shouldn’t respond to,” says Professor Michael Croft, Ph.D., Director of Scientific Affairs and Head of the Division of Immune Regulation at La Jolla Institute for Immunology (LJI).
To cure these diseases, LJI scientists need to know exactly what triggers T cells to turn on the body. This mission has led them to explore the power of genetics, cell development, and signaling molecules in one of the most complex systems in the galaxy: the human body.
What is your genetic risk?
Immunologist Pandurangan Vijayanand, M.D., Ph.D., the William K. Bowes Distinguished Professor at LJI, is putting together the genetic puzzle behind autoimmune diseases.

“We know that genes play a role in autoimmune diseases,” Dr. Vijayanand says. “If you have a twin with type 1 diabetes, for example, your risk of also having type 1 diabetes is about 50 percent—much higher than the typical risk. Scientists have also found that risk of autoimmune disease can be linked to certain populations. For example, people of African or Chinese origin have a higher risk of developing lupus.
“The challenge is to track down these risk factors in the DNA code,” he adds. “There are diseases where a single genetic mutation—one little DNA “letter” out of place—triggers disease. But few autoimmune diseases have such a simple clear genetic culprit. Instead, researchers believe subtle variations in groups of genes usually work together to increase risk.”
Many studies have compared the full genomes of people with and without autoimmune diseases. Each person will naturally have numerous genetic variations, so Dr. Vijayanand and many others are sorting through thousands of people to see which commonly found genetic variants are more prevalent in people with different diseases.
From there, Dr. Vijayanand’s team is going further to connect genetic variations to their effects in specific immune cells. Maybe a variation linked to lupus will have no effect in T cells but will be important in another immune cell type, B cells. “Once we know there’s a gene effect in a particular cell type, then people can investigate further in humans or in model organisms,” says Dr. Vijayanand.
Already, Dr. Vijayanand and his LJI colleagues have built a database called DICE (Database of Immune Cell Expression, Expression of quantitative trait loci, and Epigenomics), which scientists can use to share pieces of the genetic puzzle. In 2018, the team published data from 91 healthy donors showing profiles of genetic activity for the 15 most abundant types of immune cells found in human blood. This database gives scientists a guide for comparing healthy variations to disease-associated mutations.
“To understand autoimmunity, you need to take a fresh look at it,” says Dr. Vijayanand. “Then you are likely to find new players, new pathways.”
Why do good T cells go bad?
So your genes control how your immune system is built, but then there’s the matter of how T cells actually mature in an organ called the thymus. In adults, the thymus weighs about three grams. That’s because its glory days are over. The thymus’ real time to shine is in infancy and toddlerhood, when it weighs about 70 grams and lays like a blanket over the heart. Every pathogen is new to a baby, so the thymus works non-stop to prepare T cells for the challenges ahead.
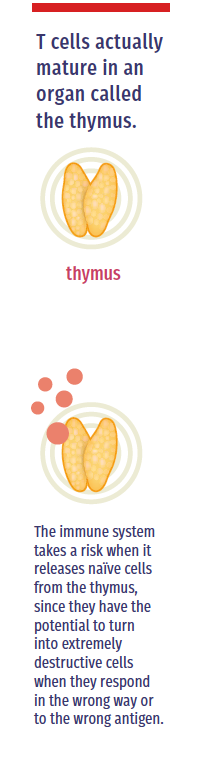
T cells that leave the thymus have shown potential, but they are untested. These cells are called naïve cells. “T cells go to school in the thymus, if you will,” says LJI President and Chief Scientific Officer Mitchell Kronenberg, Ph.D. While most come out ready to function, others “might become the T cell equivalent of juvenile delinquents.”
Dr. Kronenberg and LJI Professor Hilde Cheroutre, Ph.D., Head of the Division of Developmental Immunology, have dedicated their careers to better understanding T cells.
Dr. Cheroutre explains there is a big difference between autoimmunity and autoimmune diseases. “Autoimmunity in itself is not necessarily bad and autoreactive T cells are normally present in healthy people.” T cells in the body are looking for cellular markers called antigens. All cells have self-antigens that signal ‘hi, it’s me and I’m a healthy cell.’ An infected cell or a cancer cell will also have antigens derived from the pathogen (non-self) or tumorspecific antigens (abnormal self-antigens) that distinguish these cells from healthy cells. The presence of those foreign or abnormal antigens alerts the T cells and they fight the infection or remove the cancer cells. T cells first learn to distinguish self from non-self in the thymus, where they are tested against self-antigens. Those that do not react to those self-antigens leave the thymus as so-called naïve T cells. When these naïve T cells detect an antigen that alerts them to a threat like an infected cell, they mature and turn into protective cells that fight the infection.
“The immune system however takes a risk when it releases these naïve cells from the thymus, since they do have the potential to turn into extremely destructive cells when they respond in the wrong way or to the wrong antigen in the periphery, causing tissue destruction and autoimmune diseases,” says Dr. Cheroutre.
But everyone also has autoreactive T cells that are specifically trained in the thymus to recognize “self.” These “educated” autoreactive T cells are important players in beneficial autoimmunity by regulating immune responses and they often also emerge as critical protective cells to eliminate cancer cells or infected cells when mechanisms to alert conventional T cells fail. Still, a naïve T cell may encounter a self-antigen that it thinks is an invader. “If that happens under the wrong circumstances, that T cell can become a very dangerous autoreactive cell,” Dr. Cheroutre explains.
To better understand what goes wrong in autoimmune diseases, Dr. Cheroutre and her team are working with doctors at Rady Children’s Hospital-San Diego. When surgeons at the hospital have to perform heart surgery on young children, they must cut out a piece of the thymus to reach the heart. The surgeons leave a good part of the thymus in the patient, and Dr. Cheroutre can study those discarded pieces of thymus to learn more about the early days of T cell development.
“We hope to be able to identify autoimmune disease susceptibility in infants and even prevent autoimmune disease from ever happening,” Dr. Cheroutre says.
Hello, Antigen!
When a T cell meets another cell, it scans that cell to detect molecular flags called antigens. Your cells should all have similar antigens that signal that the cell is “self.” If a T cell encounters a “foreign” antigen it doesn’t recognize, such as from a virus or bacteria, it may sound the alarm— the body is under attack! However, signals from other cells, such as macrophages, the white blood cells that “eat” foreign substances or cells lining the mucosal tissues, are usually required with antigens to activate T cells. Cancer researchers would actually like to take advantage of antigen recognition. If they can prompt T cells to see antigens on cancer cells as foreign, they may be able to get the immune system to better fight tumors.
Why are only some tissues in the line of fire?
Autoimmune diseases have a lot in common. If something is altered in T cells and genes, it really ought to affect the whole body. This is the case for systemic autoimmune diseases, such as lupus. Yet many autoimmune diseases are known for primarily affecting just one type of tissue: the skin (psoriasis), nerve fibers (multiple sclerosis), spinal joints (ankylosing spondylitis), the tissue that lines joints (rheumatoid arthritis), and salivary and tear glands (Sjögren’s Syndrome).
To understand why so many diseases affect just one tissue, Dr. von Herrath is taking a closer look at type 1 diabetes. In this disease, self-reactive T cells kill beta cells in the pancreas, destroying the body’s ability to produce insulin. Dr. von Herrath is exploring the hypothesis that there’s something in the pancreas driving its own destruction. He’s testing the theory that there’s something wrong with the beta cells themselves. Prior to the onset of clinical signs of diabetes, the beta cells are drawing T cells to the area. These beta cells may have genetic mutations—or perhaps the cells are fighting off some type of virus.
Either way, the cells seem to want to die. Dr. von Herrath thinks understanding why could be key to unlocking the root of many autoimmune diseases.
But T cells certainly encounter other signals from inside the body. Dr. Croft is investigating how proteins called costimulatory molecules affect T cell function. Costimulatory molecules act on individual T cells, and research is indicating they play a role in causing T cells to attack tissues in autoimmune disease.


Hormones also seem to play a role. Women are more susceptible to most autoimmune diseases than men. Researchers believe this is tied to the female body’s ability to sustain a pregnancy, since pregnancy requires that the body tone down immune responses in order to not reject foreign tissue (the developing fetus). This adds a layer of complexity to the female immune system and may lead to more opportunities for T cells to attack the wrong tissues.
Researchers at LJI are working to understand the flow of signals around the body and how this affects where destructive T cells are headed.
A shared hope
Every day, LJI scientists make progress in answering the big questions about autoimmune diseases, and they find hope in recent breakthroughs.
Dr. Kronenberg thinks it’s interesting that a biologic drug shown to treat inflammatory bowel disease has also proven effective against arthritis. Bowels and joints don’t tend to have a lot in common, but in this case, the effective treatment for an autoimmune disease in one tissue also works in other tissues.
In the end, it’s more about studying what makes autoimmune diseases the same than what makes them different. “The end organ is important, but the underlying cause is immune system dysfunction, and attacking that can be most effective,” says Dr. Kronenberg.
Basic research is unlocking the secrets of autoimmune diseases, and LJI researchers have brought their expertise together to answer the biggest questions in the field. “We don’t come at the problem from one angle, and we don’t know where the next insight is going to come from,” says Dr. Croft.
“I think the future is bright,” adds Dr. Kronenberg.


