“Receiving a SPARK award was a very special event in my early scientific career. It not only gave me the confidence to think of bold ideas but also trained me to execute them within a definite time frame. I am honored to say that the SPARK award prepared me in so many ways for the next step in my scientific journey.”
What if we could cure asthma by erasing the immune system’s memory of allergens?
Funded: January 2022
Funded By: The generosity of LJI Board Director Dave Rickey and the Rickey Family
What was the goal of your SPARK project?
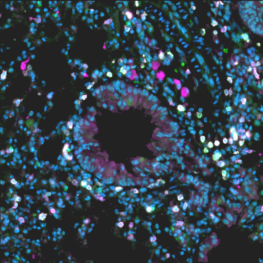
I set out to develop a mouse model to study how the immune system remembers allergens. I worked with Foxp3 GFP reporter mice subjected to the multiple exposures to house dust mites extract (human allergen), which leads to the development of memory T cells in their lungs. By staining their lung tissue with fluorochrome antibodies, I tracked down a particular subset of lung-localized memory T cells. These memory T cells were sorted out and further processed for RNA extraction and single-cell RNA sequencing. The next half of the project was critical. I aimed to analyze the single-cell RNA sequence data from these T cells using different bioinformatics tools to help us understand the allergic memory T cells. By comparing the allergic memory T cells with memory T cells of naïve mice, I hoped to shed light on the differentially expressed genes responsible for the long-lasting persistence of these allergic memory T cells.
Did you face any challenges?
Life never goes as we plan, and neither does science. Unfortunately, due to some technical issues, all the samples of naïve mice were not of good quality, so we were left with no control samples to compare our lung-localized bad memory T cells of allergic mice. However, my plan B saved the project. I managed to sort out and sequence all the memory T cells in circulation, along with lung-localized memory cells of allergic mice. Furthermore, I realized that the circulatory memory T cells of allergic mice were an excellent internal control to better understand the long-lasting behavior of tissue-resident memory T cells of allergic lungs.
SPARK project results:
My SPARK award led to the development of a mouse model for studying allergic asthma—and the discovery of this dangerous group of “memory” T cells. I used two experimental mouse model groups, a naïve group and the allergic asthma group, to track down the lung-localized T cells carrying the markers of memory T cells. By harnessing a technique called scRNA seq and using different bioinformatic tools, I found that the pool of memory T cells is not a single population but a heterogenous pool of different subsets of T cells. Interestingly, my results indicated that the pool of lung-localized memory T cells comprises more than ten different sub-population. It is essential to mention that all of these members are unique and carry different gene signatures.
What’s next for this project?
I plan to explore which subset(s) of the long-lasting memory T cells in this study would be the most notorious member(s) of this extended family. We need to figure out which of these cells has the potential to survive and thus to be responsible for therapy-resistant asthma, a severe clinical situation that needs to be addressed on an urgent basis. I am honored to have received funding for one more year to test this extended SPARK idea.
What’s next for Gurupreet?
I have been working in the field of asthma immunology for the last nine years, and the next step for me is to establish my lab in this
direction. Notably, the SPARK award helped me to explore a very less-traveled path in the field of asthma immunology; to examine lung-localized, long-lasting bad memory T cells against allergens. I strongly believe this project will help me to open more avenues in this area. In the near future, this information might help scientists identify possible targets to erase the immune system’s long-lived allergic memory and help asthma patients live healthier lives.
2022 PROGRESS PITCH WINNER:
Dr. Sethi’s initial SPARK Award allowed him to make progress in understanding asthma, leading to the development of a mouse model for studying allergic asthma—and the discovery of a dangerous group of
bad “memory” T cells.
As winner of the 2022 SPARK Progress Pitches, Dr. Sethi proposed the additional funding to figure out which memory T cells in allergic lungs are the worst. These mysterious cells can survive asthma treatments and may be responsible for the reappearance of disease symptoms—a condition referred to as therapy-resistant asthma.
What is the goal of this next phase of your SPARK Project?
Recently in the Croft lab we replicated the clinical situation of therapy-resistance in a mouse model. Now the plan is to uncover and understand the role of bad memory T cells under these complex asthma settings. Given the heterogeneous nature and long-lasting persistence of these memory T cells, I hypothesized their close connection with the reappearance of disease symptoms after stopping the therapy in severe asthma patients. I firmly believe that studying the gene profile of bad memory T cells that survived asthma treatment, and their comparison to that of the therapy-sensitive memory T cells, will take us one step closer to developing long-lasting therapies for asthma patients.
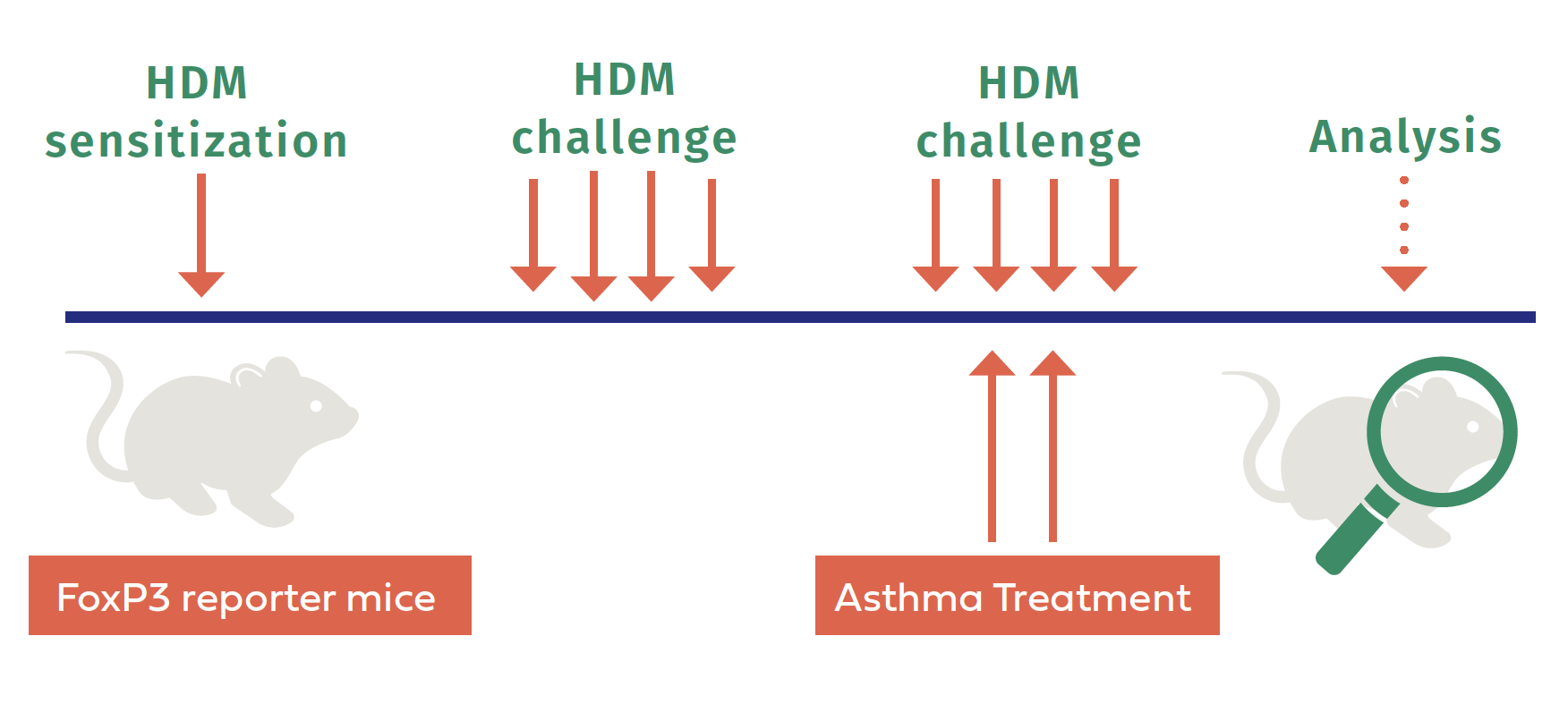
What progress have you made so far and what comes next?
So far, we have performed the main experiment by subjecting specialized mice, called “FoxP3 Reporter Mice,” to multiple exposures of a human allergen, standardized house dust mites, in order to develop the pool of bad memory T cells in their lungs (see the figure above). We have already demonstrated that modulating bad memory T cells by targeting their surface molecules, namely CD3 co-receptors, provides temporary relief to disease symptoms. Thus, I decided to treat some allergic mice with anti-CD3 F(ab’)2 antibodies to mimic the therapy-resistant-like settings in lab conditions. We then collected lung tissue, isolated the bad memory T cells, and processed them for RNA extraction. Finally, we obtained the single-cell RNA sequence data of allergic mice. My next step is to analyze the RNA sequencing data
to understand what markers of these bad memory T cells might be responsible for their long-lasting survival, even after receiving the asthma therapies. Understanding this will be helpful in managing, and perhaps even curing, severe asthma.