Overview
The LJI SURGE Program features projects that have a strong translational opportunity but need funding to bridge the gap from discovery to clinical trials/market. Philanthropic support for the SURGE fund or a specific SURGE projects gives donors the opportunity to directly contribute to bringing discoveries closer to patients.
BACKGROUND
Food allergies have been increasing at an alarming rate over the last few decades. Almost 8 percent of children under three years old and 4 percent of adults suffer from food allergies. When exposed to food allergens, most commonly peanuts, dairy, and shellfish, people with food allergies experience wheezing, itches, rashes and gastrointestinal distress—and in severe cases, deadly anaphylactic shock. Yet, despite the widespread problem, allergy treatments haven’t advanced much in decades and mostly focus on treating allergy symptoms.
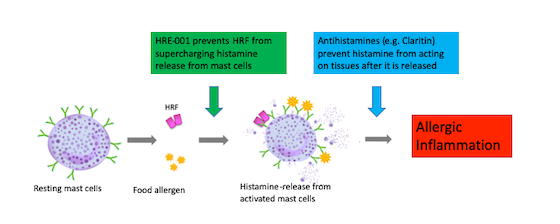
Two types of immune cells — called mast cells and basophils — are the biggest culprits behind allergic reactions. When a patient encounters an allergen, let’s say peanut protein, the allergen binds to a type of antibody called immunoglobulin E, or IgE, that have occupied its receptors on mast cells and basophils. This stimulates the mast cells and basophils to release a storm of histamine, leukotrienes, prostaglandins and ultimately cytokines that provoke an allergic response to help exorcise peanut protein from the body.
Antihistamines—such as Benadryl, Claritin, Zyrtec and others—relieve allergy symptoms by blocking histamine receptors. Xolair, an engineered antibody, binds to human IgE and thus blocks it from triggering an inflammatory response. While effective in severe allergic asthma, it takes 6 – 8 weeks to fully kick in so it cannot be used for an acute attack. Classic allergy desensitization therapy, in which patients train their immune system to ignore increasing quantities of allergen via allergy shots is not only painstakingly slow, it also causes adverse events (AEs) in up to 95 percent of patients.
HISTAMINE RELEASING FACTOR
Dr. Toshiaki Kawakami discovered that histamine-releasing factor (HRF) serves as a “food allergy amplifier” and does as its name suggests: When activated by food allergens, HRF proteins bind to IgE and then both synergistically trigger the release of histamine and other allergy mediators from mast cells and basophils and enhance inflammation.
HRE-001 AND ITS POTENTIAL USES
Dr. Kawakami developed a peptide-based inhibitor (HRE-001), that blocks the activation of HRF. When administered orally to mice allergic to egg protein, HRE-001 ameliorated food-allergy symptoms such as gut inflammation, drops in body temperature and increased release of histamine and cytokines. The team also observed higher than normal levels of IgE antibodies extremely responsive to HRF (what immunologists call “HRF-reactive IgE”) in the blood of allergic mice, very similar to what is observed in children with egg allergies. This is a clear indication that HRF contributes to food allergy in humans like it does in mice. In addition, HRF-reactive IgE levels were reduced in patients who had undergone successful oral desensitization therapy to egg protein.
When administering just HRE-001 30 minutes prior to the allergen exposure, the allergic response is ameliorated in these animals. As this agent acts prior to mast cell activation by IgE, this product can protect against histamine release. Furthermore, the peptide is relatively quick acting so if one wants to go to dinner in a place where allergen (for example, peanut) exposure is potentially high, taking HRE-001 30 minutes prior to dining out would potentially be enough to mitigate an allergic response.
Another potential use for HRE-001 is to attenuate AEs associated with allergy sensitization strategies. Once Proof of Concept is confirmed with HRE-001 in both a peanut and ova allergic mouse model, we would like to introduce it to those companies that are in the process of receiving approval for their peanut allergy sensitization regimen. The combination of these therapies has the potential of significantly reducing the 95% AE rates observed during trials and improve compliance while being treated.
Taken together, these findings make HRE-001 a compelling biological for the treatment of food allergies.
PROMISE
Since HRE-001 that shuts down the allergic cascade before it reaches mast cells and basophils, it provides an alternative for people who don’t respond to antihistamines. Unlike Xolair, which takes weeks to become effective, HRE-001 acts within 30 minutes. This holds great promise for a prophylactic pill, which, when taken before eating at public places such as the school cafeteria or restaurants, could prevent the risk of anaphylactic shock when unexpectedly encountering a potentially deadly allergen.
Most importantly, it could be given in conjunction with classic immunotherapy to control adverse reactions during the desensitization process and to speed up the timeline. Currently the process is painstakingly long—not just months, but years—to treat a single allergy and many patients suffer from multiple allergies.
BACKGROUND AND CHALLENGES OF DENGUE AND ZIKA VIRUSES
Dengue virus has been steadily expanding around the globe since the 1940s, but its close relative, Zika virus, had remained relatively unknown until the 2015/2016 outbreaks catapulted the virus into the public’s consciousness. Zika and dengue go hand in hand. Both are mosquito-borne members of the same flavivirus family that are endemic to overlapping subtropical and tropical regions. But flavivirus-carrying mosquitoes have rapidly expanded beyond their established territories in South East Asia and Latin America and have reached Southern Europe and even the continental United States, putting half the world’s population at risk of infection.
ABOUT DENGUE VIRUS:
Dengue virus infects an estimated 390 million people each year. In most cases, infection with dengue causes a mild, flu-like illness, but for reasons not yet fully understood, in some cases, the disease turns into the life-threatening severe dengue disease, which can send the victim into shock. Without an approved vaccine to stem the increasing tide of dengue infections, dengue fever is now the world’s most common mosquito-borne disease.
ABOUT ZIKA VIRUS:
Similar to dengue, manifestations of Zika infection can differ drastically. Sometimes they are catastrophic, most notably when they cause microcephaly in some babies born to infected mothers. At other times, they are mild and fleeting, suggesting that complex virus-host interactions temper Zika-induced disease severity.
A COMPLICATED RELATIONSHIP:
Recently, prior exposure to dengue virus emerged as a key factor that determines the severity of Zika infection—not only because dengue is endemic in most of the regions affected by Zika’s global spread, but also because both viruses shares genetic and structural characteristics that allows the immune system to recognize Zika almost like another version of dengue. However, while a prior dengue infection may have a protective effect against Zika (and vice versa), the opposite can be true as well due to the process known as antibody-dependent enhancement (ADE). In ADE, cross-reactive antibodies—that are either non-neutralizing or sub-neutralizing—help viruses slip into certain cells, paradoxically increasing rather than blocking the viral infection. This effect may explain why no vaccine to dengue has been successful despite 70 years of efforts. Given the geographic co-mingling of dengue virus and Zika virus, any successful vaccine candidate needs to avoid a potential ADE response by targeting both viruses simultaneously.
KEY DISCOVERY
Dr. Sujan Shresta’s laboratory was one of the first to demonstrate the importance of cross-reactive T cells to induce protective immunity and counterbalance the presence of ADE-mediating antibodies. The group’s recent data have shown that a solid T cell response also confers protection against Zika infection in mice. Based on these pioneering insights into the mechanisms of pathology and immunity of dengue and Zika, Dr. Shresta is developing a pan-flavivirus vaccine that elicits both neutralizing Ab and protective T cell responses against all 4 dengue serotypes as well as Zika without triggering ADE. This novel approach will solve the longstanding dengue and Zika vaccine obstacle.
HOW DOES IT WORK AND WHAT’S NEXT?
Proof of concept data will be obtained by using DNA-based vaccine candidates that specifically drive the expression of both antibody and T cell-targeting proteins of all 4 dengue serotypes as well as Zika. Immunodominant epitopes that are recognized by ADE-causing antibodies will be mutated. This novel vaccine design is thus expected to induce both humoral and cellular immunity against dengue and Zika while avoiding ADE.
The immunogenicity and protective efficacy of vaccine will be evaluated first in wild-type mice. As the vaccine is DNA-based, an electroporator is required to perform DNA injection into mice. Key results based on studies with wild-type mice will be validated using type I interferon receptor-deficient mice, which unlike wild-type mice are highly susceptible to dengue and Zika infection.
LIFE WITHOUT DENGUE AND ZIKA
With the generation of proof-of-concept data for a universal flavivirus vaccine by the end of one year, we anticipate that our intellectual property will be significantly enhanced and commercial interest considerable. This vaccine for prevention of dengue (DENV) and Zika (ZIKV) infections with maximal safety and efficacy has the potential to stop enormous health and economic burdens associated with these virus infections by saving millions of lives and billions of dollars.
