Allergies
Conditions such as eczema, contact dermatitis, allergy-induced asthma and hay fever are all connected to allergy.
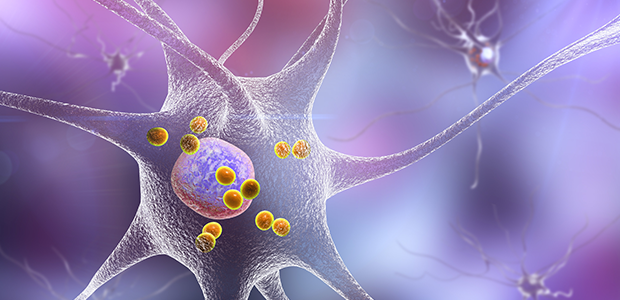
La Jolla Institute scientists are working to understand how the body's T cells may target the proteins involved in the plaques and tangles seen in brains with Alzheimer's disease.
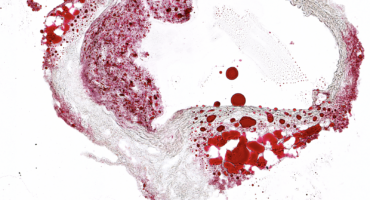
The underlying cause of many heart attacks is atherosclerosis, defined as build-up of deposits, or plaques, of cholesterol, calcium, and other substances in arteries.
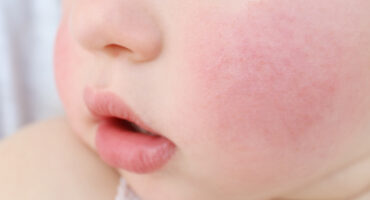
Atopic dermatitis occurs when a person’s immune system causes inflammation of the skin, which also makes the skin more vulnerable to environmental irritants and allergens.
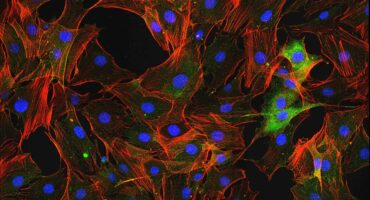
Scientists at La Jolla Institute are investigating what causes immune cells to turn on the body—and how we can intervene.
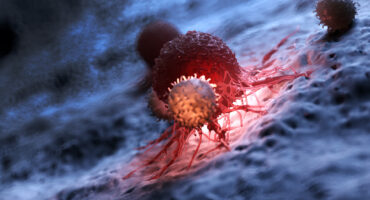
One in four deaths in the United States is caused by cancer. Scientists at La Jolla Institute for Immunology (LJI) are committed to making cancer immunotherapies work for more people.
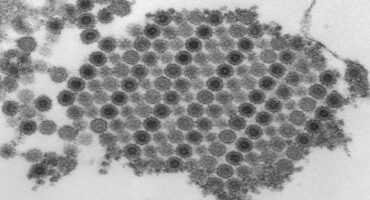
Researchers at La Jolla Institute for Immunology (LJI) are investigating how CHIKV infects cells and how the immune system responds to a CHIKV attack.

Allergens like egg, shellfish, or peanut proteins stimulate white blood cells to dump excessive quantities of immune molecules into the blood, triggering inflammation.
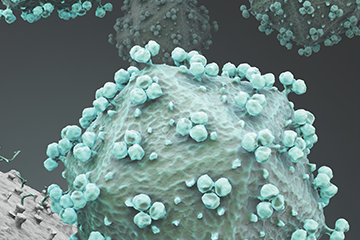
La Jolla Institute scientists are working to help develop an HIV/AIDS vaccine that can stimulate the immune system to neutralize many strains of the virus.
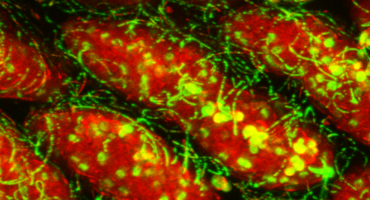
Researchers at La Jolla Institute are working to prevent the symptoms of IBD before inflammation escalates.
More than 3 billion people are at risk of Japanese encephalitis, a viral disease that sickens an estimated 68,000 people each year, killing up to 20,400.
Lassa virus is endemic to West Africa and kills nearly 5,000 people a year. Research led by La Jolla Institute scientists lays the groundwork for anti-Lassa vaccine development.
Lung cancer is the leading cause of cancer death for both men and women in the United States. Researchers at La Jolla Institute for Immunology are studying ways to halt lung cancer growth.
Researchers at La Jolla Institute are investigating how the immune system may contribute to Parkinson's disease.

Our Approach The recent increase in Powassan cases—and the potential for infection to cause serious symptoms—has led scientists at La
Millions of people suffer from seasonal allergies. For them, the onset of spring signals several months of misery.
Strep throat is one of a several conditions, among them pneumonia, scarlet fever, impetigo, and flesh-eating necrotizing fasciitis, caused by the bacterium Streptococcus pyogenes.
Tuberculosis kills more people each year than any other infectious disease. Scientists at La Jolla Institute are working to help doctors treat those at most risk—sooner.
Type 1 diabetes accounts for up to 2 million U.S. cases and occurs when the immune system mistakenly destroys insulin-producing cells in the pancreas.

Vasculitis is the umbrella term for nearly 20 rare diseases all associated with inflammation of the blood vessels.
This extremely contagious disease is caused by the bacterium Bordetella pertussis, and it can be fatal in newborns.
The yellow fever vaccine is one of vaccine biology’s great success stories. Scientists are hoping to learn how to design effective vaccines against other pathogens by studying the immune response induced by the yellow fever vaccine.